Covid Vaccines Overview
1 School of Anthropology University of Arizona. All charts and tables below reflect the data release on 8062021 from the VAERS website which includes US.

Ziad Khatib Msc Emba Phd Assoc Prof בטוויטר Nice Overview Bbcnews On Coronavirus Vaccines Comparing The Different Companies And The Explaining The Vaccine Process Vaccineswork Https T Co Ttgmrguasx Https T Co Xfszcv7u87
Only 18 of people in low-income countries have received at least one dose.
Covid vaccines overview
. This attenuated organism acts as an antigen and stimulates the. The use of vaccines has prevented countless cases of infections preventing suffering and saving millions of lives. Conventional vaccine types include the following. Overview Vaccines are free and widely available.2 Mel and Enid Zuckerman College of Public Health. Since 2020 vaccine development has been expedited via unprecedented collaboration. COVID-19 vaccine efficacy summary. An overview of COVID-19 vaccines their distribution and acceptance and hesitation.
COVID-19s caused virus SARS-CoV-2 severe acute respiratory syndrome coronavirus 2 was isolated in late 2019. An immediate allergic reaction means a reaction within 4 hours of getting the shot including symptoms such as hives swelling or wheezing respiratory distress. COVID-19 vaccines are medicines that prevent disease caused by the novel coronavirus SARS-CoV-2 by triggering an immune response. Note that the total number of deaths associated with the COVID-19 vaccines is greater than the number of deaths associated with.
Moderna - Doses Two doses are needed 28 days apart or up to six weeks apart if needed in the US. EMA releases a monthly update for each authorised COVID-19 vaccine. 544 billion doses have been administered globally and 3564 million are now administered each day. VAERS Summary for COVID-19 Vaccines through 7302021.
To be updated periodically. To project future COVID-19 trends IHME centralizes and updates all available data on vaccine efficacy. Implement routine and emergency procedures for the proper administration monitoring storage and disposal of vaccines. All currently authorized and recommended COVID-19 vaccines are safe and effective and CDC does not recommend one vaccine over another.
They also indicate whether any safety information requires further investigation. If you received a Pfizer-BioNTech or Moderna COVID-19 vaccine you. Some protection provided after the first dose. Learn about common side effects of COVID-19 vaccines and when to call a doctor.
1 Joseph Fong MPH. Live-attenuated vaccines such as the measles-mumps-rubella vaccine contain attenuated weakened forms of an organism that causes a disease. And foreign data and is updated through. The safety updates summarise the data that have become available since the vaccines authorisation.
2 Amy Lind. This document summarizes the available data and key underlying assumptions of IHMEs projections. 402 of the world population has received at least one dose of a COVID-19 vaccine. Nichter PhD MPH.
Discuss COVID-19 vaccine emergency use authorization EUD development and safety protocols. All charts and tables below reflect the data release on 8062021 from the VAERS website which includes US. Its genetic sequence was published on 11 January 2020 triggering an urgent international response to prepare for an outbreak and hasten development of a preventive COVID-19 vaccine. You dont need health insurance to get vaccinated for COVID-19.
The CDC recommends a third dose of an mRNA COVID-19 vaccine at least 28 days after the second dose for some people with weakened immune systems such as those who have had an organ transplant. COVID-19 vaccines are not interchangeable. Note that the total number of deaths associated with the COVID-19 vaccines is greater than the number of deaths associated with all other vaccines combined since the year 1990. Analyze the standard infection prevention precautions and vaccine preparation protocols in order to reduce risk of contamination.
2 Collin Catalfamo MPH. Everyone in the United States ages 12 and older is eligible to receive free COVID-19 vaccines regardless of immigration status. EMA publishes safety updates for the COVID-19 vaccines authorised in the EU. COVID-19 Vaccine Overview Vaccines are important public health tools that can prevent many different bacterial and viral infections.
Safe and effective vaccines for COVID-19 are needed because they protect individuals from becoming ill. And foreign data and is updated through.

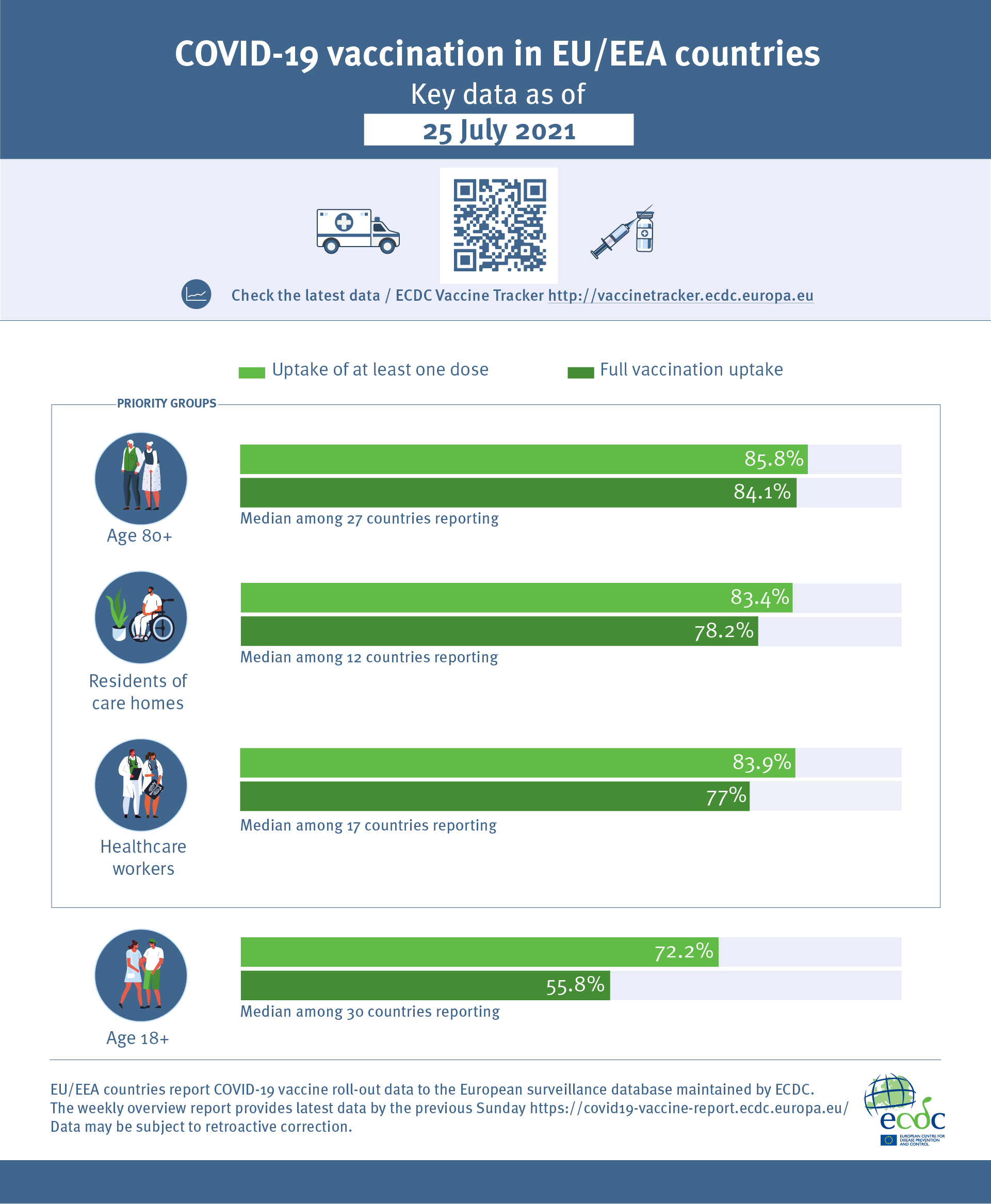
Infographic Covid 19 Vaccination In The Eu Eea As Of 25 July 2021

166 Vaccines In The Race Against Covid 19 Germany Among The Frontrunners Avertim

Vaccine Overview Issue 4 Cido Ontology Cido Github

Covid 19 Eu Global Vaccine Solidarity Consilium
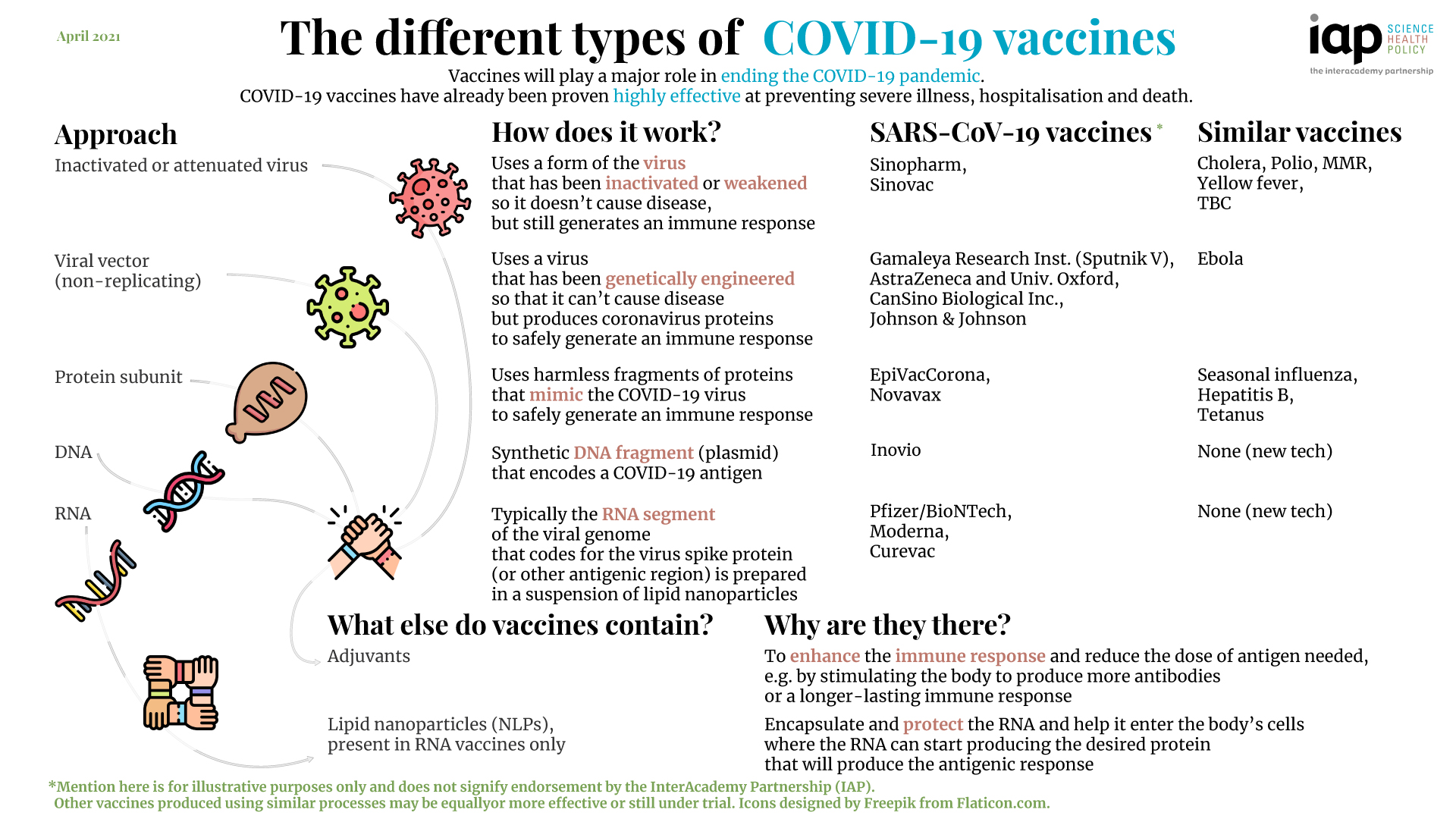
The Different Types Of Covid 19 Vaccines

Covid 19 The Eu S Emergency Response Consilium

Meps Urge Eu Countries To Be Transparent About Their Covid 19 Vaccine Supplies News European Parliament
Its genetic sequence was published on 11 January 2020 triggering an urgent international response to prepare for an outbreak and hasten development of a preventive COVID-19 vaccine. The safety updates summarise the data that have become available since the vaccines authorisation.

Ziad Khatib Msc Emba Phd Assoc Prof בטוויטר Nice Overview Bbcnews On Coronavirus Vaccines Comparing The Different Companies And The Explaining The Vaccine Process Vaccineswork Https T Co Ttgmrguasx Https T Co Xfszcv7u87
Since 2020 vaccine development has been expedited via unprecedented collaboration.

Covid vaccines overview
. The use of vaccines has prevented countless cases of infections preventing suffering and saving millions of lives. You dont need health insurance to get vaccinated for COVID-19. 2 Mel and Enid Zuckerman College of Public Health. Note that the total number of deaths associated with the COVID-19 vaccines is greater than the number of deaths associated with.If you received a Pfizer-BioNTech or Moderna COVID-19 vaccine you. 1 Joseph Fong MPH. Some protection provided after the first dose. Live-attenuated vaccines such as the measles-mumps-rubella vaccine contain attenuated weakened forms of an organism that causes a disease.
An overview of COVID-19 vaccines their distribution and acceptance and hesitation. Safe and effective vaccines for COVID-19 are needed because they protect individuals from becoming ill. Discuss COVID-19 vaccine emergency use authorization EUD development and safety protocols. Overview Vaccines are free and widely available.
To project future COVID-19 trends IHME centralizes and updates all available data on vaccine efficacy. Implement routine and emergency procedures for the proper administration monitoring storage and disposal of vaccines. All currently authorized and recommended COVID-19 vaccines are safe and effective and CDC does not recommend one vaccine over another. Note that the total number of deaths associated with the COVID-19 vaccines is greater than the number of deaths associated with all other vaccines combined since the year 1990.
EMA releases a monthly update for each authorised COVID-19 vaccine. COVID-19 vaccine efficacy summary. EMA publishes safety updates for the COVID-19 vaccines authorised in the EU. All charts and tables below reflect the data release on 8062021 from the VAERS website which includes US.
Learn about common side effects of COVID-19 vaccines and when to call a doctor. They also indicate whether any safety information requires further investigation. Conventional vaccine types include the following. This attenuated organism acts as an antigen and stimulates the.
Nichter PhD MPH. An immediate allergic reaction means a reaction within 4 hours of getting the shot including symptoms such as hives swelling or wheezing respiratory distress. COVID-19 vaccines are not interchangeable. 2 Collin Catalfamo MPH.
COVID-19 vaccines are medicines that prevent disease caused by the novel coronavirus SARS-CoV-2 by triggering an immune response. Analyze the standard infection prevention precautions and vaccine preparation protocols in order to reduce risk of contamination. 2 Amy Lind. This document summarizes the available data and key underlying assumptions of IHMEs projections.
To be updated periodically. The CDC recommends a third dose of an mRNA COVID-19 vaccine at least 28 days after the second dose for some people with weakened immune systems such as those who have had an organ transplant. COVID-19 Vaccine Overview Vaccines are important public health tools that can prevent many different bacterial and viral infections. Moderna - Doses Two doses are needed 28 days apart or up to six weeks apart if needed in the US.
Everyone in the United States ages 12 and older is eligible to receive free COVID-19 vaccines regardless of immigration status. 544 billion doses have been administered globally and 3564 million are now administered each day. VAERS Summary for COVID-19 Vaccines through 7302021. 402 of the world population has received at least one dose of a COVID-19 vaccine.
And foreign data and is updated through. And foreign data and is updated through. COVID-19s caused virus SARS-CoV-2 severe acute respiratory syndrome coronavirus 2 was isolated in late 2019.

Covid 19 Eu Global Vaccine Solidarity Consilium

166 Vaccines In The Race Against Covid 19 Germany Among The Frontrunners Avertim

Meps Urge Eu Countries To Be Transparent About Their Covid 19 Vaccine Supplies News European Parliament

Vaccine Overview Issue 4 Cido Ontology Cido Github

Infographic Covid 19 Vaccination In The Eu Eea As Of 25 July 2021
Posting Komentar untuk "Covid Vaccines Overview"